Does Buckminsterfullerene Conduct Electricity? A Comprehensive Analysis
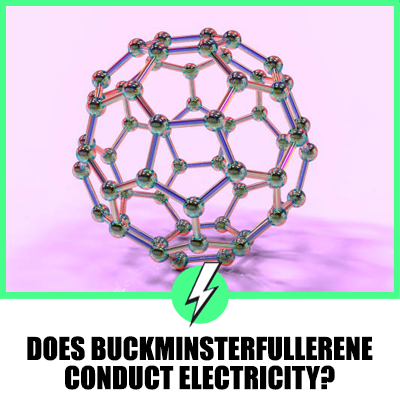
Buckminsterfullerene, also known as C60 or Buckyball, is a fascinating molecule that has intrigued scientists since its discovery.
Its unique structure, resembling a soccer ball with a pattern of 20 hexagons and 12 pentagons, has led to numerous studies exploring its properties.
One of the most frequently asked questions is whether buckminsterfullerene conducts electricity.
This article aims to answer this question and provide a comprehensive understanding of Buckminsterfullerene’s electrical properties.
Contents
Why is Buckminsterfullerene a Good Conductor of Electricity?
The answer to this question is not straightforward.
Buckminsterfullerene is not a good conductor of electricity in its pure form.
This is because each carbon atom in C60 is bonded to three other carbon atoms, leaving no free electrons to carry an electric charge.
However, when C60 is doped with certain other elements, it can become conductive.
This is because the added atoms can donate or accept electrons, creating charge carriers within the C60 structure.
Why Does Buckminsterfullerene Not Conduct Electricity?
In its pure form, buckminsterfullerene does not conduct electricity because it lacks free electrons.
Each carbon atom in C60 is bonded to three other carbon atoms, forming a stable structure with no free electrons.
Furthermore, the structure of C60 is not conducive to the flow of electricity.
The carbon atoms are arranged in a spherical shape, with each atom participating in three bonds.
This leaves no room for the movement of electrons, which is necessary for electrical conductivity.
Is Buckminsterfullerene an Insulator or Conductor?
In its pure form, buckminsterfullerene is an insulator.
It does not conduct electricity because it lacks free electrons and its structure does not allow for the movement of electrons.
However, when doped with certain other elements, C60 can become a conductor.
The added atoms can donate or accept electrons, creating charge carriers within the C60 structure.
Is Buckyball a Conductive?
Buckyball, another name for buckminsterfullerene, is not conductive in its pure form.
It becomes conductive only when doped with certain other elements.
Insights from Online Discussions
Online discussions provide a wealth of information and diverse perspectives on the electrical conductivity of buckminsterfullerene.
Here are some insights gleaned from these discussions:
- Quora Discussion: A user on Quora explains that buckminsterfullerene does not conduct electricity because each carbon atom is bonded to three other carbon atoms, leaving no free electrons to carry an electric charge. However, when doped with certain other elements, C60 can become conductive.
- Reddit Discussion: A Reddit user points out that while each carbon atom in C60 is bonded to three other carbon atoms, the structure of C60 is not conducive to the flow of electricity. The carbon atoms are arranged in a spherical shape, with each atom participating in three bonds. This leaves no room for the movement of electrons, which is necessary for electrical conductivity.
- Byjus Discussion: A user on Byjus agrees that buckminsterfullerene does not conduct electricity in its pure form. However, when doped with certain other elements, C60 can become a conductor.
- Chemistry Stack Exchange Discussion: A user on Chemistry Stack Exchange provides a detailed explanation of why C60 is an insulator. They explain that the carbon atoms in C60 are bonded in a way that creates a stable structure with no free electrons. Furthermore, the structure of C60 is not conducive to the flow of electricity.
- Faculty.csbsju.edu Article: An article on faculty.csbsju.edu provides a detailed explanation of the bonding and electronic structure of buckminsterfullerene. It explains that each carbon atom in C60 forms three sigma bonds with its sp2 hybrid orbitals and one pi bond with the remaining p-orbital. This structure is not conducive to the flow of electricity.
Implications for the UK and US Audience
For both the UK and US audience, understanding the electrical properties of Buckminsterfullerene is crucial.
In both countries, Buckminsterfullerene is widely used in a variety of applications, from research to industrial uses.
The fact that it is an electrical insulator in its pure form but can become a conductor when doped with certain elements is important for safety reasons.
For instance, in the electronics industry, Buckminsterfullerene can be used to insulate electrical components, preventing short circuits and other electrical issues.
This is particularly important in the UK and US, where safety standards for electrical products are stringent.
Furthermore, the knowledge that Buckminsterfullerene can become conductive when doped with certain elements can open up new possibilities for researchers and professionals in both countries.
These conductive properties can be used in applications where traditional conductive materials are not suitable, providing a versatile solution for a wide range of electrical projects.
Conclusion
In conclusion, Buckminsterfullerene does not conduct electricity in its pure form.
It becomes conductive only when doped with certain other elements.
This fascinating molecule continues to be a subject of research, and future studies may reveal more about its unique properties.
As we continue to use and understand these substances, it’s important to keep in mind their properties and the implications of these properties on their use and safety.